The platform is equipped with complete hardware and software resources, experienced research talents and advanced solid-state research and analysis instruments, such as HPLC, GC, Lc-mass, Pre-HPLC, etc., which can carry out quality research on APIs in the following aspects.
According to national pharmacopoeia standards or self-developed analytical methods for the detection of various items, mainly including the following categories.
|
Physical Constant |
Melting point, density, pH, spin, conductivity and TOC |
|
Identify |
UV, IR, HPLC, etc. |
|
Limits |
Moisture, dry weight loss, residue scorch, total ash, elemental impurities, residual solvents and related substances |
|
Property Check |
Appearance color, solution color, solution clarity, visible foreign matter, etc. |
|
Content |
Content determination (including chromatographic, volumetric and gravimetric methods) |
|
Solid State Analysis |
Crystalline shape, particle size, bulk density, bulk density and rheological fluidity, etc. |
Main analytical instruments are:
|
Chromatography |
HPLC(VWD、DAD)、Gas Chromatograph(FID) |
|
Spectroscopy |
IR、UV |
|
Mass Spectrometry |
HPLC-mass、GC-mass、ICP-mass |
|
Titration |
Potentiometric titrator, Karl Fischer moisture titrator |
|
Physical and Chemical |
Melting point meter, pH meter, balance |
|
Solid State |
PSD、XRD、DSC、TGA |
Based on ICH guidelines and the requirements of national pharmacopoeias, the development, confirmation and validation of analytical methods for elemental impurities in APIs provide the basis for risk assessment of elemental impurities in APIs, mainly using inductively coupled plasma mass spectrometry (ICP-mass).
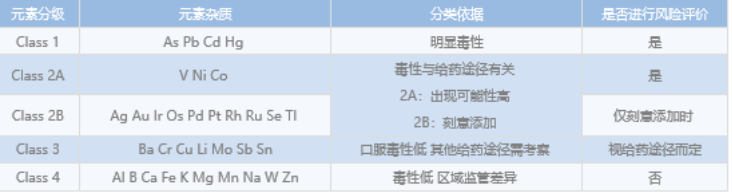
In recent years, national regulatory agencies such as ICH, FDA, EMA, etc. have had clearer requirements for genotoxic impurities, and more and more pharmaceutical companies are focusing on the control and detection of genotoxic impurities during the development of new drugs according to ICH M7 "Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk". Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk" for genotoxic impurities. Depending on the limits of genotoxic impurities, they can be performed using UPLC/UV, UPLC/QDa (single quadrupole), and other instruments, respectively.
Stability research is based on the systematic study and understanding of the API or formulation and its production process, through the design of tests to obtain the quality characteristics of the API or formulation under the influence of various environmental factors (such as temperature, humidity, light exposure, etc.) over time, and accordingly for the drug prescription, process, packaging, storage conditions and retest period / expiration date to provide supporting information; including impact factor tests It includes influence factor tests, accelerated tests and long-term retention tests. The platform has equipment such as Mimert stability test chambers.


